Place:
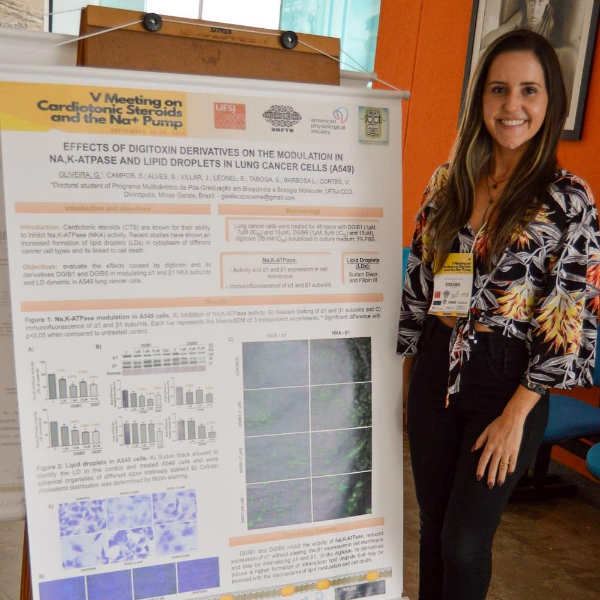
All biology depends first upon chemistry. The mammalian brain, especially that of humans, is the most sophisticated biological structure known to man utilizing a massive array of chemical entities. Steroids are a crucial class of chemical entities within the CNS. They impact numerous functions including mood, sexual orientation, memory, blood pressure, salt and water balance, etc. Included among these steroids are endogenous cardiotonic steroids (CTS) including ouabain – ordinarily considered to be a CTS of plant origin. Ouabain and its endogenous mammalian counterpart (EO) are highly polar CTS that are structurally identical but with different origins. Under normal conditions (intact blood brain barrier), neither has free access to the CNS. However, both can enter the hypothalamus in limited amounts via the fenestrated epithelia surrounding the circumventricular organs of the hypothalamus. This region is heavily invested in the control of water and electrolyte balance, sympathetic nerve activity and long-term blood pressure control. Within the CNS, EO-like materials are highly enriched in discrete hypothalamic neurons that project from the supraoptic and paraventricular nuclei to the rostral ventral medulla and pituitary. The hypothalamic (and pituitary) content of EO represents a mix of local hypothalamic synthesis plus that coming via the circulation from the adrenal glands (Hamlyn et al, 2014, Leenen et al, 2020). Elsewhere in the CNS, under normal circumstances, most of the CNS EO arises from local synthesis. Within the brain, there are polymorphic receptors for EO as reflected by the sodium pump α subunit isoforms. But are there polymorphic ligands for these sodium pump isoforms? Many classic mammalian steroids have numerous isomeric analogs but, thus far, no naturally occurring isomers of ouabain in the plant kingdom have been described. Are there isomers of EO in mammals and if so where are they and what do they do? Some answers to these questions arose by accident during an investigation of the effects of CNS angiotensin II (Ang II) on long-term blood pressure in rodents. It is well accepted that sustained increases in CNS Ang II stimulate angiotensin II type I receptors (AT1R), activate the sympathetic nervous system and cause hypertension. Remarkably, the mechanism by which this occurs involves aldosterone and EO synthesis in the CNS. Blockade of aldosterone receptors as well inhibition of aldosterone synthesis in the brain prevents hypertension and returns the elevated circulating EO (measured by routine radioimmunoassay (RIA)) to normal. When the RIA measurements were repeated after HPLC to confirm that EO per se was the basis of the immunocrossreactivity, two immunoreactive peaks in addition to EO were detected. These were proven subsequently to be EO isomers by multidimensional mass spectrometry. Isomer 1 was more polar than EO and minimally impacted by CNS blockade of aldosterone. In contrast, isomer 2 was less polar than EO, was sensitive to CNS Ang II and aldosterone receptor blockade, but was increased >30 fold in the circulation of animals in which the brain synthesis of aldosterone was suppressed by fadrazole. Thus, this work demonstrates that the circulation of rodents contains multiple ligands patterned on EO that, together with multiple Na pump isoforms, add to the already wide biological panorama in this arena. In contrast to the periphery, the CNS is a privileged space surrounded by a blood brain barrier. In the CNS large chemical gradients can occur as the result of local biosynthesis. To determine whether EO and these isomers are present in discrete regions in the CNS, fresh adult bovine brains were interrogated by direct electrospray mass spectrometry. EO and isomer 2 were found throughout the CNS but not always together. Moreover, five discrete “hot areas” were observed for EO where its concentrations were 10-100x higher than the surrounding tissue. Two hot areas were found for isomer 2, only one of which overlapped with EO. Hot areas were especially prominent in the hypothalamus, brain stem and the hippocampus. The observation that these isomers are present in rodents and cattle suggests they are of widespread significance in mammals and likely play a targeted role in Na pump regulation and signaling especially in discreet brain regions. Several of these EO enriched areas are in locations relevant to CNS conditions including mania and bipolar disorders, depression, epilepsy, and memory. All of these hot areas have been linked previously with both beneficial as well as adverse dose-dependent actions of EO, ouabain and other CTS. Further, the hypothalamic and brain stem sites have obvious and direct relevance to the long term hypertensinogenic actions of ouabain and EO already documented by many studies. The interactions of isoforms and isomers and their impact on biology in health and disease represent a new chapter in the book of biology.
Presented by Dr. Luciana Rossoni